Potassium Acetate USP BP Ph Eur Reagent Grade & Potassium Citrate USP BP IP Ph Eur Reagent FCC Food Grade Suppliers Exporters, Manufacturers
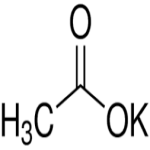
Potassium Acetate
CAS Number: 127-08-2 USP BP Ph Eur Reagent Grade Suppliers Exporters, Manufacturers

Please visit Safety Data Sheet of Potassium Acetate USP BP Ph Eur Reagent Grade Manufacturers.
Potassium Acetate USP Grade Specifications:
C2H3KO2 --- 98.14
Acetic acid, potassium salt
Potassium acetate CAS 127-08-2
DEFINITION
Potassium Acetate contains NLT 99.0% and NMT 100.5% of C2H3KO2, calculated on the dried basis.
IDENTIFICATION
A. Identification Tests—General, Potassium.
B. Identification Tests—General, Acetate.
ASSAY
Procedure
Sample: 200 mg of Potassium Acetate, previously dried
Analysis: Dissolve the Sample in 25 mL of glacial acetic acid, add 2 drops of crystal violet and titrate with 0.1 N perchloric acid to a green endpoint. Perform a blank determination and make any necessary correction. Each mL of 0.1 N perchloric acid is equivalent to 9.814 mg of potassium acetate (C2H3KO2).
Acceptance criteria: 99.0% to 100.5% on the dried basis
pH:
Sample solution: 50 mg/mL
Acceptance criteria: 7.5 to 8.5
Loss on Drying: Dry a sample at 150C for 2 h: it loses NMT 1.0% of its weight.
Packaging and Storage: Preserve in tight containers.
Specifications of Potassium Acetate BP Ph Eur Grade
C2H3O2K -- 98.14 -- 127-08-2
DEFINITION
Potassium acetate contains not less than 99.0 per cent and not more than the equivalent of 101.0 per cent of C2H3KO2, calculated with reference to the dried substance.
CHARACTERS
A white, crystalline powder or colourless crystals, deliquescent, very soluble in water, freely soluble in alcohol.
IDENTIFICATION
A. It gives reaction of acetates.
B. It gives reaction of potassium.
TESTS
Solution S: Dissolve 10.0 g in distilled water and dilute to 100 ml with the same solvent.
Appearance of solution: Solution S is clear and colourless.
pH: Dissolve 1.0 g in carbon dioxide-free water and dilute to 20 ml with the same solvent. The pH of the solution is 7.5 to 9.0.
Reducing substances: Dilute 10 ml of solution S to 100 ml with water. Add 5 ml of dilute sulphuric acid and 0.5 ml of a 0.32 g/l solution of potassium permanganate. Mix and boil gently for 5 min. The solution remains pink.
Chlorides: Dilute 2.5 ml of solution S to 15 ml with water. The solution complies with the limit test for chlorides (200 ppm).
Sulphates: Dilute 7.5 ml of solution S to 15 ml with distilled water. The solution complies with the limit test for sulphates (200 ppm).
Aluminium: If intended for use in the manufacture of peritoneal dialysis solutions, haemofiltration solutions or hem dialysis solutions, it complies with the test for aluminium. Dissolve 2.0 g in 50 ml of water and add 5 ml of acetate buffer solution pH 6.0. The solution complies with the limit test for aluminium (1 ppm). Use as a reference solution a mixture of 1 ml of aluminium standard solution (2 ppm Al), 5 ml of acetate buffer solution pH 6.0 R and 49 ml of water. To prepare the blank use a mixture of 5 ml of acetate buffer solution pH 6.0 R and 50 ml of water.
Iron: Dilute 5 ml of solution S to 10 ml with water. The solution complies with the limit test for iron (20 ppm).
Heavy metals: Dissolve 5.0 g in water and dilute to 20 ml with the same solvent. 12 ml of the solution complies with limit test A for heavy metals (4 ppm). Prepare the standard using lead standard solution (1 ppm Pb).
Sodium: Not more than 0.5 per cent of Na, determined by atomic emission
Loss on drying: Not more than 3.0 per cent, determined on 1.000 g by drying in an oven at 100C to 105C.
Potassium Acetate Analytical Reagent Grade Specifications
CH3COOK
Formula Weight. 98.14
CAS Number 127-08-2
REQUIREMENTS
Assay: 99.0% CH3COOK
pH of a 5% solution: 6.5-9.0 at 25C
MAXIMUM ALLOWABLE
Insoluble matter: 0.005%
Chloride (Cl): 0.003%
Phosphate (PO4): 0.001%
Sulfate (SO4): 0.002%
Heavy metals (as Pb): 5 ppm
Iron (Fe): 5 ppm
Calcium (Ca): 0.005%
Magnesium (Mg): 0.002%
Sodium (Na): 0.03%
Please visit Hazard Statement of Potassium Acetate USP BP Ph Eur Reagent Grade Manufacturers.
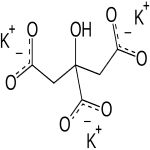
Potassium Citrate
CAS Number: 6100-05-6 Monohydrate & 866-84-2 Anhydrous USP BP IP Ph Eur Reagent FCC Food Grade Suppliers Exporters, Manufacturers

Potassium Citrate USP Grade Specifications:
C6H5K3O7-H2O --- 324.41
C6H5K3O7 --- 306.40
1,2,3-Propanetricarboxylic acid, 2-hydroxy-, tripotassium salt, monohydrate;
Tripotassium citrate monohydrate CAS 6100-05-6
Potassium Citrate Anhydrous CAS 866-84-2.
DEFINITION
Potassium Citrate contains NLT 99.0% and NMT 100.5% of potassium citrate (C6H5K3O7), calculated on the dried basis.
IDENTIFICATION
A. Identification Tests: General, Potassium and Citrate: A 100-mg/mL solution meets the requirements.
ASSAY
Procedure
Sample: 200 mg of Potassium Citrate
Blank: 25 mL of glacial acetic acid
Titrimetric system
Mode: Direct titration
Titrant: 0.1 N perchloric acid
Endpoint detection: Visual
Analysis: Dissolve the Sample in 25 mL of glacial acetic acid, add 2 drops of crystal violet TS, and titrate with the Titrant to a green endpoint. Perform a Blank determination.
Calculate the percentage of potassium citrate (C6H5K3O7) in the Sample taken:
Result = {[( V S − V B ) × N × F]/W} × 100
V S = Titrant volume consumed by the Sample (mL)
V B = Titrant volume consumed by the Blank ;(mL)
N = actual normality of the Titrant (mEq/mL)
F = equivalency factor, 102.1 mg/mEq
W = Sample weight (mg)
Acceptance criteria: 99.0% to 100.5% on the dried basis
Sample: 1 g of Potassium Citrate
Analysis: Dissolve the Sample in 1.5 mL of water in a test tube, add 1 mL of 6 N acetic acid, and scratch the walls of the test tube with a glass rod.
Acceptance criteria: No crystalline precipitate is formed.
Alkalinity:
Sample solution: Dissolve 1 g of Potassium Citrate in 20 mL of water.
Analysis: To the Sample solution add 0.20 mL of 0.10 N sulfuric acid, and add 1 drop of phenolphthalein.
Acceptance criteria: The Sample solution is alkaline to litmus. No pink color is produced after the addition of phenolphthalein.
Loss on Drying: Dry a sample at 180C for 4 h: it loses 3.0% to 6.0% of its weight.
Packaging and Storage: Preserve in tight containers.
Potassium Citrate BP Ph Eur Grade Specifications
C6H5K3O7-H2O -- 324.4 -- CAS 6100-05-6
DEFINITION
Potassium citrate contains not less than 99.0 per cent and not more than the equivalent of 101.0 per cent of tripotassium 2-hydroxypropane-1,2,3-tricarboxylate, calculated with reference to the anhydrous substance.
CHARACTERS
A white, granular powder or transparent crystals, hygroscopic, very soluble in water, practically insoluble in alcohol.
IDENTIFICATION
A. To 1 ml of solution S (see Tests) add 4 ml of water. The solution gives the reaction of citrates.
B. 0.5 ml of solution S gives reaction of potassium.
TESTS
Solution S: Dissolve 10.0 g in carbon dioxide-free water prepared from distilled water and dilute to 100 ml with the same solvent.
Appearance of solution: Solution S is clear and colourless.
Acidity or alkalinity: To 10 ml of solution S add 0.1 ml of PhPh solution. Not more than 0.2 ml of 0.1 M hydrochloric acid or 0.1 M sodium hydroxide is required to change the colour of the indicator.
Readily carbonizable substances: To 0.20 g of the powdered substance to be examined add 10 ml of sulphuric acid and heat in a water-bath at 90 ± 1C for 60 min. Cool rapidly. The solution is not more intensely coloured than reference solution.
Chlorides: Dilute 10 ml of solution S to 15 ml with water. The solution complies with the limit test for chlorides (50 ppm).
Oxalates: Dissolve 0.50 g in 4 ml of water, add 3 ml of hydrochloric acid and 1 g of granulated zinc and heat on a water-bath for 1 min. Allow to stand for 2 min, decant the liquid into a test-tube containing 0.25 ml of a 10 g/l solution of phenylhydrazine hydrochloride and heat to boiling. Cool rapidly, transfer to a graduated cylinder and add an equal volume of hydrochloric acid and 0.25 ml of potassium ferricyanide solution. Shake and allow to stand for 30 min. Any pink colour in the solution is not more intense than that in a standard prepared at the same time and in the same manner using 4 ml of a 0.05 g/l solution of oxalic acid (300 ppm).
Sulphates: To 10 ml of solution S add 2 ml of hydrochloric acid and dilute to 15 ml with distilled water. The solution complies with the limit test for sulphates (150 ppm).
Heavy metals: 12 ml of solution S complies with limit test A for heavy metals (10 ppm).
Sodium: Not more than 0.3 per cent of Na, determined by atomic emission spectrometry.
Water: 4.0 per cent to 7.0 per cent, determined on 0.500 g by the semi-micro determination of water. After adding the substance to be examined, stir for 15 min before titrating.
Specifications of Potassium Citrate IP Grade
Potassium citrate IP grade is also offered.
Specifications of Potassium Citrate FCC Food Grade
Tripotassium Citrate
KOOCCH2C(OH)(COOK)CH2COOK-H2O
C6H5K3O7-H2O Formula weightt 324.41
INS: 332(ii) CAS: 6100-05-6
DESCRIPTION
Potassium Citrate occurs as transparent crystals or as a white, granular powder. It is deliquescent when exposed to moist air. One gram dissolves in about 0.5 mL of water. It is almost insoluble in alcohol.
Function: Buffer; sequestrant; stabilizer.
REQUIREMENTS
Identification: A 1:20 aqueous solution gives positive tests for Potassium and for Citrate.
Assay: Not less than 99.0% and not more than 100.5% of C6H5K3O7 after drying.
Alkalinity: A 1:20 aqueous solution is alkaline to litmus, but after the addition of 0.2 mL of 0.1 N sulfuric acid to 10 mL of this solution, no pink color appears after the addition of 1 drop of PhPh.
Lead: Not more than 2 mg/kg.
Loss on Drying: Between 3.0% and 6.0%.
We manufacture and supply Micro Encapsulated Potassium Citrate. The core consists of pure grade of 80% Potassium Citrate and the coating will be of 20% polymer. Encapsulation makes the core material slow release or extended release.
We also manufacture Micro-encapsulated Potassium Citrate.
Potassium Acetate CAS Number 127-08-2 & Potassium Citrate CAS Number 6100-05-6 Monohydrate & 866-84-2 Anhydrous Supplier Exporter, Manufacturer:
Annie Chemie P Ltd
Mumbai 4000010, INDIA
With Agents and offices in UAE, USA, Europe.
e-mail: info@anniechemie.com
Copyright and Usual Disclaimer is Applicable.
June 1, 2025
Exporters to USA, Canada, UK, Europe, UAE, Nigeria, Algeria, Turkey, Mexico, Brazil, Chile, Argentina, Australia, Dubai etc.
Perfection is made up of small things and that is a big thing.
