Monopotassium Phosphate Dipotassium Phosphate Tripotassium Phosphate Potassium Phosphate Monobasic Dibasic Tribasic USP NF BP Ph Eur Reagent FCC Food Grade Suppliers Exporters, Manufacturers
Monopotassium Phosphate or Potassium Phosphate Monobasic
CAS Number: 7778-77-0 USP NF BP Ph Eur Reagent FCC Food Grade Suppliers Exporters, Manufacturers

Monobasic Potassium Phosphate USP NF Grade Specifications:
KH2PO4 --- 136.09
Phosphoric acid, monopotassium salt;
Monopotassium phosphate CAS 7778-77-0
DEFINITION
Monobasic Potassium Phosphate, dried at 105C for 4 h, contains NLT 98.0% and NMT 100.5% of monobasic potassium phosphate (KH2PO4).
IDENTIFICATION
A. Identification Tests-General, Potassium
B. Identification Tests-General, Phosphate
ASSAY
Procedure
Sample solution: Transfer 5 g of Monobasic Potassium Phosphate, previously dried, to a 250-mL beaker. Add 100 mL of water and 5.0 mL of 1 N hydrochloric acid and stir until the assay specimen is completely dissolved.
Titrimetric system
Mode: Direct titration
Titrant: 1 N sodium hydroxide
Endpoint detection: Potentiometrically
Analysis: Slowly titrate the excess acid in the Sample solution, stirring constantly, with Titrant to the inflection point occurring at about pH 4 (V S1). Continue the titration with Titrant until the inflection point occurring at about pH 8.8 is reached (V S2).
Calculate the percentage of monobasic potassium phosphate (KH2PO4) in the sample taken:
Result = {[(V S2 - V S1) x N x F]/W} x 100
V S2 = Titrant volume consumed by the Sample solution to the second inflection point (mL)
V S1 = Titrant volume consumed by the Sample solution to the first inflection point (mL)
N = actual normality of the Titrant (mEq/mL)
F = equivalency factor, 0.1361 g/mEq
W = weight of monobasic potassium phosphate taken to prepare the Sample solution (g)
Acceptance criteria: 98.0% to 100.5% on the previously dried basis.
Lead:
Test preparation: 1 g in 20 mL of water
Acceptance criteria: NMT 5 µg/g
Limit of Fluoride: To pass the test.
Acceptance criteria: NMT 10 µg/g
Insoluble Substances:
Sample solution: 10 g in 100 mL of hot water
Analysis: Filter the Sample solution through a tared filtering crucible, wash the insoluble residue with hot water, and dry at 105C for 2 h.
Acceptance criteria: NMT 20 mg (0.2%)
Loss on Drying:
Analysis: Dry a sample at 105C for 4 h.
Acceptance criteria: NMT 1.0%
Packaging and Storage: Preserve in tight containers.
Specifications of Potassium Dihydrogen Phosphate BP Ph Eur Grade
Monopotassium Phosphate BP
Potassium Phosphate Monobasic BP
KH2PO4 -- 136.1 -- CAS 7778-77-0
Monobasic Potassium Phosphate
Potassium Phosphate Monobasic
DEFINITION
Potassium dihydrogen phosphate contains not less than 98.0 per cent and not more than the equivalent of 100.5 per cent of KH2PO4, calculated with reference to the dried substance.
CHARACTERS
A white, crystalline powder or colourless crystals, freely soluble in water, practically insoluble in alcohol.
IDENTIFICATION
A. Solution S (see Tests) is faintly acid.
B. Solution S gives reaction (b) of phosphates.
C. 0.5 ml of solution S gives reaction of potassium.
TESTS
Solution S: Dissolve 10.0 g in carbon dioxide-free water prepared from distilled water and dilute to 100 ml with the same solvent.
Appearance of solution: Solution S is clear and colourless.
pH: To 5 ml of solution S add 5 ml of carbon dioxide-free water. The pH of the solution is 4.2 to 4.5.
Reducing substances: To 5 ml of solution S add 5 ml of dilute sulphuric acid and 0.25 ml of 0.02 M potassium permanganate. Heat on a water-bath for 5 min. The colour of the permanganate is not completely discharged.
Chlorides: Dilute 2.5 ml of solution S to 15 ml with water R. The solution complies with the limit test for chlorides (200 ppm).
Sulphates: To 5 ml of solution S add 0.5 ml of hydrochloric acid and dilute to 15 ml with distilled water. The solution complies with the limit test for sulphates (300 ppm).
Arsenic: 0.5 g complies with limit test A for arsenic (2 ppm).
Iron: 10 ml of solution S complies with the limit test for iron (10 ppm).
Sodium: If intended for use in the manufacture of parenteral dosage forms, it complies with the test for sodium. Not more than 0.1 per cent of Na, determined by atomic emission spectrometry
Heavy metals: 12 ml of solution S complies with limit test A for heavy metals (10 ppm).
Loss on drying: Not more than 2.0 per cent, determined on 1.000 g by drying in an oven at 125C to 130C.
Potassium Phosphate Monobasic FCC Food Grade
Potassium Biphosphate; Potassium Dihydrogen Phosphate
Monopotassium Phosphate FCC
Monobasic Potassium Phosphate FCC
KH2PO4 Formula weight 136.09
INS: 340(i) CAS: 7778-77-0
DESCRIPTION
Potassium Phosphate, Monobasic, occurs as colorless crystals or as a white, granular or crystalline powder. It is stable in air. It is freely soluble in water, but is insoluble in alcohol. The pH of a 1:100 aqueous solution is between 4.2 and 4.7.
Function: Buffer; sequestrant; yeast food.
REQUIREMENTS
Identification: A 1:20 aqueous solution gives positive tests for Potassium and for Phosphate.
Assay: Not less than 98.0% of KH2PO4 after drying.
Arsenic: Not more than 3 mg/kg.
Fluoride: Not more than 10 mg/kg.
Insoluble Substances: Not more than 0.2%.
Lead: Not more than 2 mg/kg.
Loss on Drying: Not more than 1%.
Specifications of Potassium Phosphate Monobasic Analytical Reagent Grade
Potassium Dihydrogen Phosphate
Monopotassium Phosphate
Monobasic Potassium Phosphate
KH2PO4
Formula weight 136.09
CAS Number 7778-77-0
REQUIREMENTS
Assay: 99.0% KH2PO4
pH of a 5% solution: 4.1-4.5 at 25C
MAXIMUM ALLOWABLE
Insoluble matter: 0.01%
Loss on drying at 105C: 0.2%
Chloride (Cl): 0.001%
Nitrogen compounds (as N): 0.001%
Sulfate (SO4): 0.003%
Heavy metals (as Pb): 0.001%
Iron (Fe): 0.002%
Sodium (Na): 0.005%.
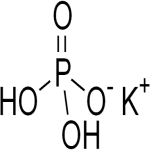
Please visit Hazard Statement of Monopotassium Phosphate or Potassium Phosphate Monobasic USP NF BP Ph Eur Reagent FCC Food Grade Suppliers.
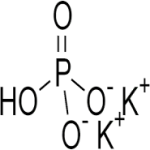
Dipotassium Phosphate or Potassium Phosphate Dibasic
CAS Number: 7758-11-4 USP BP Ph Eur Reagent FCC Food Grade Suppliers Exporters, Manufacturers

Dibasic Potassium Phosphate USP Grade Specifications:
K2HPO4 --- 174.18
Phosphoric acid, dipotassium salt;
Dipotassium hydrogen phosphate CAS 7758-11-4
DEFINITION
Dibasic Potassium Phosphate contains NLT 98.0% and NMT 100.5% of K2HPO4, calculated on the dried basis.
IDENTIFICATION
A. Identification Tests-General, Potassium
B. Identification Tests-General, Phosphate
ASSAY
Procedure
Sample: 5.2 g
Sample solution: Transfer the Sample to a 250-mL beaker. Add 50 mL of water and 40.0 mL of 1 N hydrochloric acid and stir until dissolved.
Blank: Transfer 40.0 mL of 1 N hydrochloric acid to a 250-mL beaker. Add 50 mL of water.
Analysis: Titrate the Blank with 1 N sodium hydroxide and record the volume of 1 N sodium hydroxide consumed. Titrate the excess acid in the Sample solution with 1 N sodium hydroxide to the inflection point at pH 4 and record the burette reading. Subtract this burette reading from that of the blank and designate the volume of 1 N sodium hydroxide resulting from this subtraction as A. Continue the titration with 1 N sodium hydroxide to the inflection point at pH 8.8, record the burette reading, and calculate the volume (B) of 1 N sodium hydroxide required in the titration between the two inflection points (pH 4 to 8.8). Where A is equal to or less than B, each mL of the volume A of 1 N sodium hydroxide is equivalent to 174.2 mg of K2HPO4. Where A is greater than B, each mL of the volume 2B - A of 1 N sodium hydroxide is equivalent to 174.2 mg of K2HPO4.
Acceptance criteria: 98.0%-100.5% on the dried basis.
Sample solution: Dissolve 10 g in 100 mL of hot water.
Analysis: Filter the Sample solution through a tared filtering crucible, wash the insoluble residue with hot water, and dry at 105C for 2 h.
Acceptance criteria: The weight of the residue is NMT 20 mg (NMT 0.2%).
Carbonate:
Sample: 1 g
Analysis: Add 3 mL of water and 2 mL of 3 N hydrochloric acid to the Sample.
Acceptance criteria: NMT a few bubbles are evolved.
Chloride and Sulfate, Chloride:
Sample: 1.0 g
Acceptance criteria: Shows no more chloride than corresponds to 0.40 mL of0.020 N hydrochloric acid (NMT 0.03%)
Chloride and Sulfate, Sulfate:
Sample: 0.20 g
Acceptance criteria: Shows no more sulfate than corresponds to 0.20 mL of 0.020 N sulfuric acid (NMT 0.1%)
Arsenic: NMT 3 ppm
Iron:
Sample solution: Dissolve 0.33 g in 10 mL of water.
Analysis: To the Sample solution add 6 mL of hydroxylamine hydrochloride solution (1 in 10) and 4 mL of orthophenanthroline solution prepared by dissolving 1 g of orthophenanthroline in 1000 mL of water containing 1 mL of 3 N hydrochloric acid, and dilute with water to 25 mL.
Acceptance criteria: Any red color produced within 1 h is not darker than that of a control prepared from 1 mL of Standard Iron Solution: NMT 30 ppm.
Sodium: A solution (1 in 10) tested on a platinum wire imparts no pronounced yellow color to a nonluminous flame.
Limit of Fluoride: To pass the test.
Acceptance criteria: NMT 0.001%
Limit of Monobasic or Tribasic Salt:
Sample solution: Dissolve 3 g in 30 mL of water, and cool to 20C.
Analysis: Add 3 drops of thymol blue to the Sample solution.
Acceptance criteria: A blue color is produced, which is changed to yellow (with a greenish tinge) by the addition of NMT 0.4 mL of 1 N hydrochloric acid.
pH: 8.5-9.6, in a solution (1 in 20)
Loss on Drying: Dry a sample at 105C to constant weight: it loses NMT 1.0% of its weight.
Packaging and Storage: Preserve in well-closed containers.
Specifications of Dipotassium Hydrogen Phosphate BP Ph Eur Grade
Dipotassium Phosphate BP Ph Eur
K2HPO4 -- 174.2 -- CAS 7758-11-4
Dibasic Potassium Phosphate
Potassium Phosphate Dibasic
DEFINITION
Dipotassium phosphate contains not less than 98.0 per cent and not more than the equivalent of 101.0 per cent of K2HPO4, calculated with reference to the dried substance.
CHARACTERS
A white powder or colourless crystals, very hygroscopic, very soluble in water, very slightly soluble in alcohol.
IDENTIFICATION
A. Solution S (see Tests) is slightly alkaline
B. Solution S gives reaction of phosphates.
C. Solution S gives reaction of potassium.
TESTS
Solution S: Dissolve 5.0 g in distilled water and dilute to 50 ml with the same solvent.
Appearance of solution: Solution S is clear and colourless.
Reducing substances: Heat on a water-bath for 5 min a mixture of 5 ml of solution S, 5 ml of dilute sulphuric acid and 0.25 ml of 0.02 M potassium permanganate. The solution remains faintly pink.
Chlorides: To 2.5 ml of solution S add 10 ml of dilute nitric acid and dilute to 15 ml with water. The solution complies with the limit test for chlorides (200 ppm).
Sulphates: To 1.5 ml of solution S add 2 ml of dilute hydrochloric acid and dilute to 15 ml with distilled water. The solution complies with the limit test for sulphates (0.1 per cent).
Arsenic: 5 ml of solution S complies with limit test A for arsenic (2 ppm).
Iron: 10 ml of solution S complies with the limit test for iron (10 ppm).
Heavy metals: Dissolve 2.0 g in 8 ml of water. Acidify with about 6 ml of dilute hydrochloric acid (pH 3 to 4) and dilute to 20 ml with water. 12 ml of the solution complies with limit test A for heavy metals (10 ppm).
Sodium: If intended for use in the manufacture of parenteral dosage forms, it contains not more than 0.1 per cent of Na, determined by atomic emission spectrometry.
Loss on drying: Not more than 2.0 per cent, determined on 1.000 g by drying in an oven at 125C to 130C.
Bacterial endotoxins: Less than 1.1 IU/mg, if intended for use in the manufacture of parenteral dosage forms without a further appropriate procedure for the removal of bacterial endotoxins.
Specifications of Potassium Phosphate Dibasic FCC Food Grade
Dipotassium Monophosphate, Dipotassium Phosphate FCC Food Grade
Dibasic Potassium Phosphate FCC
K2HPO4 Formula wt 174.18
INS: 340(ii) CAS: 7758-11-4
DESCRIPTION
Potassium Phosphate, Dibasic, occurs as a colorless or white, granular salt that is deliquescent when exposed to moist air. One gram is soluble in about 3 mL of water. It is insoluble in alcohol. The pH of a 1% solution is about 9.
Function: Buffer; sequestrant; yeast food.
REQUIREMENTS
Identification: A 1:20 aqueous solution gives positive tests for Potassium and for Phosphate.
Assay: Not less than 98.0% of K2HPO4 after drying.
Arsenic: Not more than 3 mg/kg.
Fluoride: Not more than 10 mg/kg.
Insoluble Substances: Not more than 0.2%.
Lead: Not more than 2 mg/kg.
Loss on Drying: Not more than 2.0%.
Specifications of Potassium Phosphate Dibasic Analytical Reagent Grade
Dibasic Potassium Phosphate
Dipotassium Phosphate
Dipotassium Hydrogen Phosphate
K2HPO4
Formula Wt 174.18
CAS Number 7758-11-4
REQUIREMENTS
Assay: 98.0% K2HPO4
pH of a 5% solution: 8.5-9.6 at 25C
MAXIMUM ALLOWABLE
Insoluble matter: 0.01%
Loss on drying at 105C: 1.0%
Chloride (Cl): 0.003%
Nitrogen compounds (as N): 0.001%
Sulfate (SO4): 0.005%
Heavy metals (as Pb): 5 ppm
Iron (Fe): 0.001%
Sodium (Na): 0.05%.
Please visit Hazard Statement of Dipotassium Phosphate or Potassium Phosphate Dibasic USP NF BP Ph Eur Reagent FCC Food Grade Suppliers.
Tripotassium Phosphate or Potassium Phosphate Tribasic
CAS Number: 7778-53-2 Analytical Reagent FCC Food Grade Suppliers Exporters, Manufacturers

Please visit Safety Data Sheet of Tripotassium Phosphate or Potassium Phosphate Tribasic Analytical Reagent FCC Food Grade Manufacturers.
Potassium Phosphate, Tribasic FCCFood Grade
Tripotassium Phosphate FCC Food Grade Specifications
Tribasic Potassium Phosphate FCC
K3PO4 Formula wt 212.27
INS: 340(iii) CAS: 7778-53-2
DESCRIPTION
Potassium Phosphate, Tribasic, occurs as white, hygroscopic crystals or granules. It is anhydrous or may contain one molecule of water of hydration. It is freely soluble in water, but is insoluble in alcohol. The pH of a 1:100 aqueous solution is about 11.5.
Function: Emulsifier.
REQUIREMENTS
Identification: A 1:20 aqueous solution gives positive tests for Potassium and for Phosphate.
Assay: Not less than 97.0% of K3PO4, calculated on the ignited basis.
Arsenic: Not more than 3 mg/kg.
Fluoride: Not more than 10 mg/kg.
Insoluble Substances: Not more than 0.2%.
Lead: Not more than 2 mg/kg.
Loss on Ignition Anhydrous: Not more than 5.0%; Monohydrate: Between 8.0% and 20.0%.
Potassium Phosphate Tribasic Analytical Reagent Grade Specifications
Tripotassium Orthophosphate
Tripotassium Phosphate Reagent
Tribasic Potassium Phosphate Reagent
K3PO4
Formula Wt 212.27
K3PO4-7H2O
Formula Wt 338.38
CAS Number 7778-53-2 anhydrous; 22763-02-6 heptahydrate
REQUIREMENTS
Assay (as-is basis): 98% K3PO4 or K3PO4-7H2O
MAXIMUM ALLOWABLE
Dibasic potassium phosphate (K2HPO4) 1%
Excess alkali (as KOH): 1%
Insoluble matter: 0.01%
Chloride (Cl): 0.005%
Nitrogen compounds (as N): 0.002%
Heavy Metals (as Pb): 0.002%
Iron (Fe): 0.001%
Sulfate (SO4): 0.005%
Sodium (Na): 0.1%.
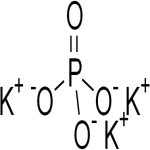
Please visit Hazard Statement of Tripotassium Phosphate or Potassium Phosphate Tribasic Analytical Reagent FCC Food Grade Suppliers.
Monopotassium Phosphate or Potassium Phosphate Monobasic CAS Number 7778-77-0, Dipotassium Phosphate or Potassium Phosphate Dibasic CAS Number 7758-11-4 & Tripotassium Phosphate or Potassium Phosphate Tribasic CAS Number 7778-53-2 Supplier Exporter, Manufacturer:
Annie Chemie P Ltd
Mumbai 4000010, INDIA
With Agents and offices in UAE, USA, Europe.
e-mail: info@anniechemie.com
Copyright and Usual Disclaimer is Applicable.
June 3, 2025
Exporters to USA, Canada, UK, Europe, UAE, Nigeria, Algeria, Turkey, Mexico, Brazil, Chile, Argentina, Australia, Dubai etc.
Perfection is made up of small things and that is a big thing.
